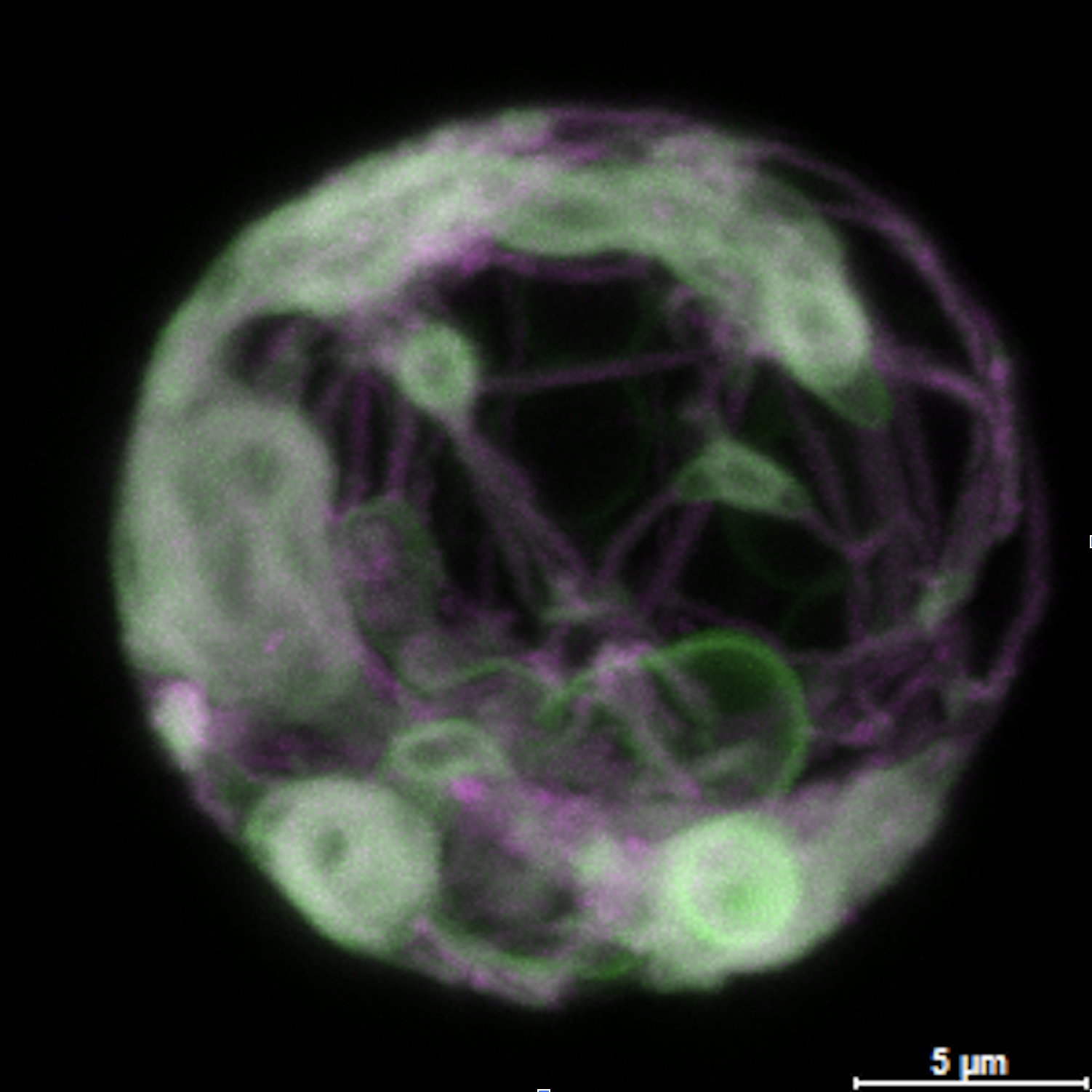
SbMSBP1 is an ER-localized MAPR protein in sorghum that binds heme, undergoes heme-dependent dimerization, and promotes membrane remodeling, suggesting roles in vesicle trafficking, cytochrome P450 stabilization, and stress-adaptive specialized metabolism.
Keywords: ER membrane dynamics, Organized smooth endoplasmic reticulum, Sorghum bicolor, membrane steroid binding protein, salt stress, vesicle transport
Characterization of the stress-responsive SbMSBP1 as a multifunctional protein connecting vesicle dynamics and metabolic enzyme organization highlights MSBPs as new targets for engineering resilient crops. – Laursen
Membrane steroid binding proteins (MSBPs) are members of the membrane-associated progesterone receptor (MAPR) family, which is conserved across eukaryotes and implicated in plant growth, stress tolerance, and steroid-related signaling. In plants, MSBPs have been associated with photomorphogenesis, brassinosteroid signaling, vesicle trafficking, and stabilization of cytochrome P450 enzymes. Scientists at University of Copenhagen and Lund University conducted a phylogenetic analysis and identified six MAPR proteins in sorghum and revealed distinct plant-specific clades, with MSBPs uniquely possessing transmembrane anchors, while other MAPR subclasses likely function in secretion or poorly understood membrane-associated processes. Sorghum MSBP1 (SbMSBP1) was found to be strongly enriched in styrene maleic acid lipid particles (SMALPs) containing enzymes of the dhurrin biosynthetic pathway, suggesting a potential role in specialized metabolism. These findings establish a framework for understanding MAPR diversification and functional specialization in plants.
Biophysical characterization demonstrated that SbMSBP1 binds ferric heme and undergoes heme-dependent homodimerization, a property previously reported only for animal MAPR homologs. Heme binding appears reversible and may regulate protein function rather than electron transfer. Contrary to earlier reports for Arabidopsis MSBP1, purified apo-SbMSBP1 showed no detectable progesterone binding, suggesting either species-specific steroid preferences or dependence on heme or membrane context. Confocal microscopy revealed localization of SbMSBP1 to the endoplasmic reticulum (ER), where its expression induced ER-derived vesicles resembling organized smooth ER (OSER) structures. Proteomics and imaging data implicate SbMSBP1 in vesicular trafficking and membrane remodeling, processes linked to stress adaptation and P450 stabilization. Together, these results identify SbMSBP1 as a multifunctional ER-associated protein with roles in heme binding, membrane organization, and potentially metabolic engineering for improved stress resilience in crops.
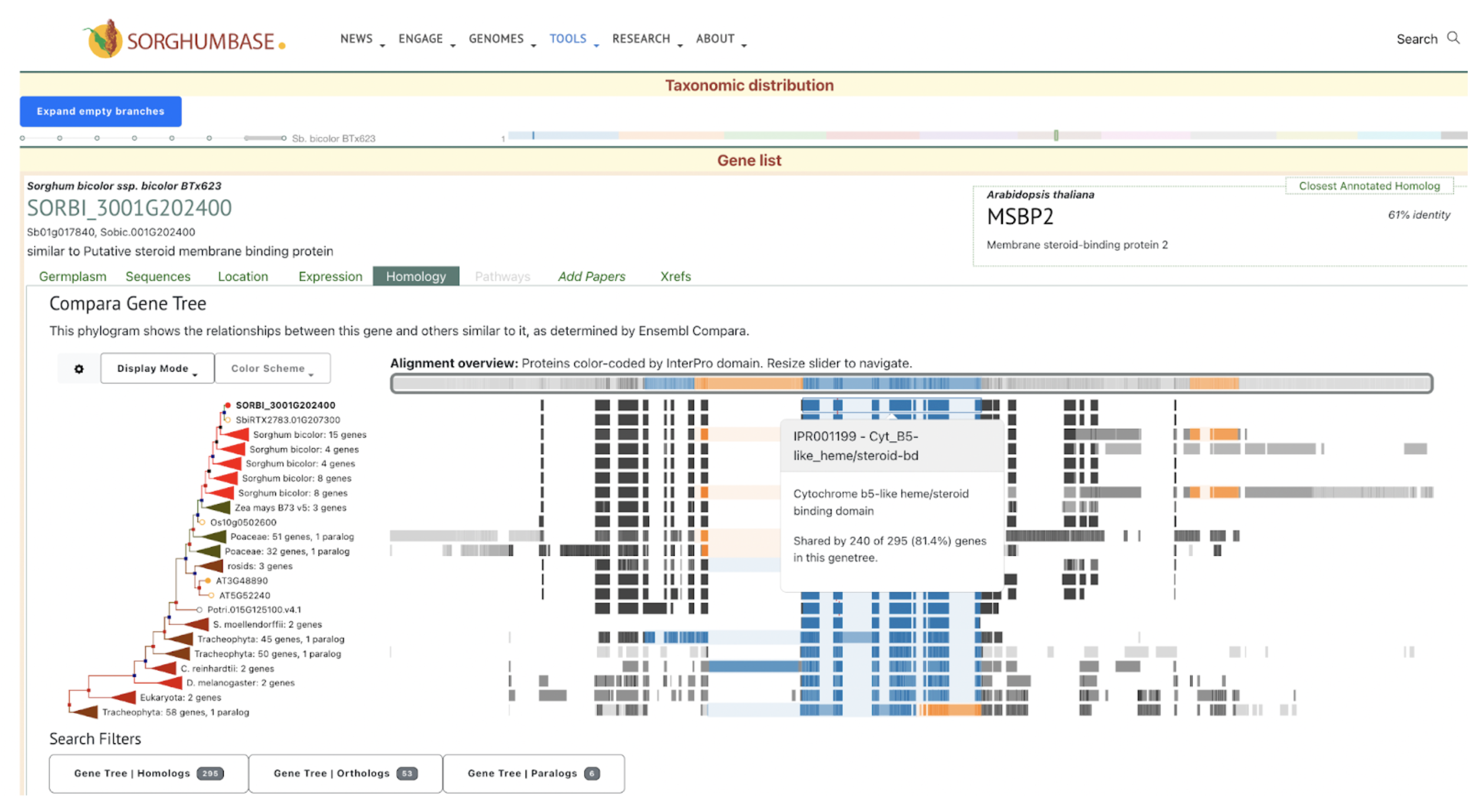
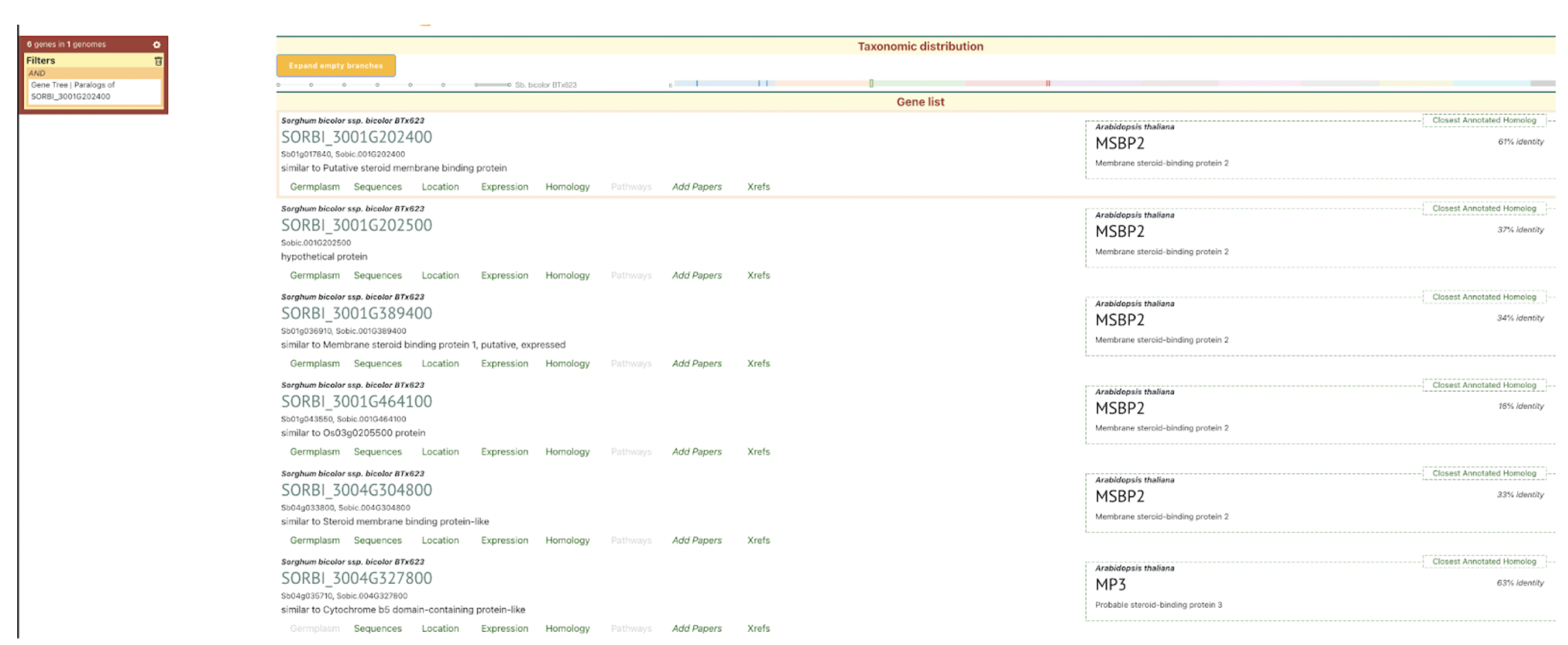
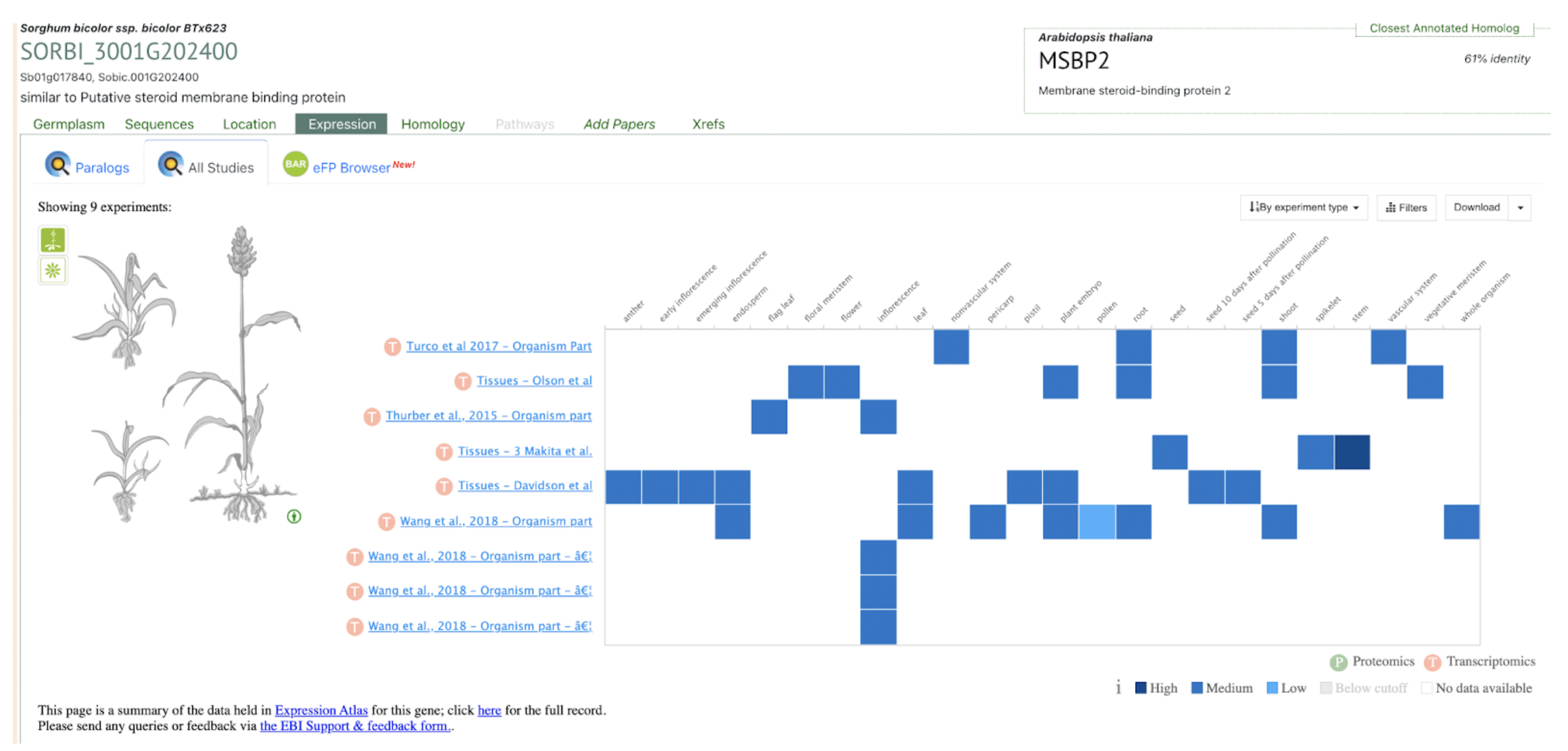
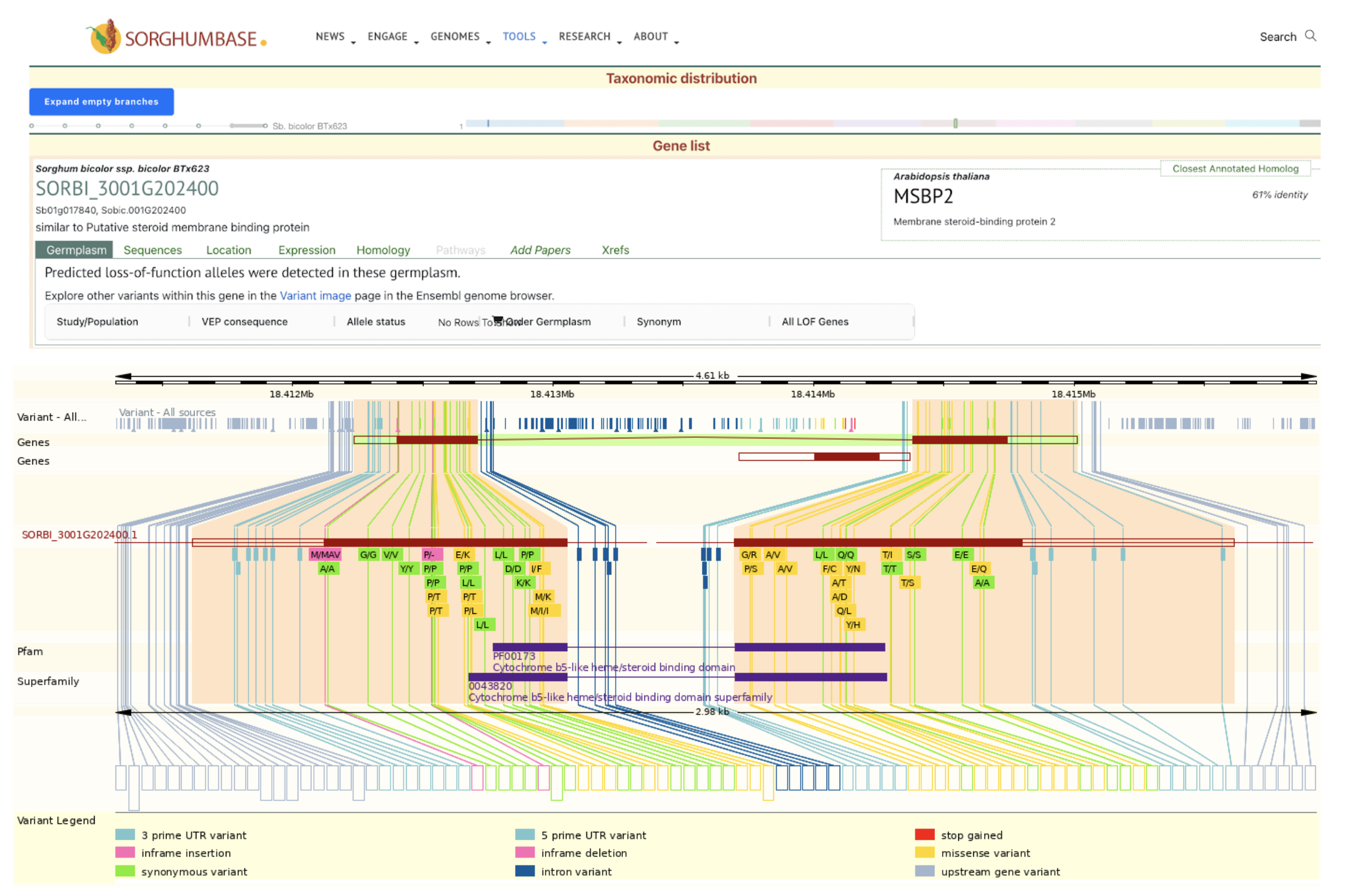
SorghumBase examples:
Data and Views from Sorghumbase search interface for gene SORBI_3001G202400 identified as SbMSBP1. https://sorghumbase.org/genes?idList=SORBI_3001G202400
The supplementary table1 of this publication lists the uniprot accession for the SbMSBP1 gene as C5WWE9. From the uniprot page https://www.uniprot.org/uniprotkb/C5WWE9/entry, we identified the standard sorghumbase gene name to be SORBI_3001G202400.
Reference:
Ratanasopa K, Ochoa-Fernandez R, Mellor SB, Travaglia V, Hinz K, Tuelung PS, Laursen T. Sorghum bicolor Membrane Steroid Binding Protein 1 can bind heme and remodels ER membranes. Plant Cell Physiol. 2025 Dec 4:pcaf160. PMID: 41342392. doi: 10.1093/pcp/pcaf160. Read more
Related Project Websites:
- Laursen Lab page at University of Copenhagen: https://plen.ku.dk/english/research/plant_biochemistry/dynamic-metabolons/