Synthetic apomixis was successfully engineered in hybrid sorghum, enabling clonal seed formation that preserves hybrid heterozygosity across generations, although further optimization is needed to improve fertility and developmental stability.
Keywords: self‐reproducing hybrids, sorghum, synthetic apomixis
This study demonstrates that synthetic apomixis can be successfully induced in sorghum, allowing hybrid plants to reproduce clonally through seed while maintaining their genetic identity across generations. Although further refinement is needed, the ability to preserve hybrid vigor has the potential to drive meaningful advances in sorghum productivity and agricultural sustainability. – Simon
Sexual reproduction in flowering plants generates genetically diverse seeds through recombination and gamete fusion; however, some species can produce clonal seeds asexually via apomixis which preserves the maternal genotype. Although apomixis is widespread in many wild plant species, it is largely absent from major crops, and the efforts to introgress the trait through conventional breeding have been largely unsuccessful. Synthetic apomixis therefore represents a transformative strategy for fixing hybrid vigor by enabling clonal seed formation, thereby preserving complete maternal heterozygosity across generations. Recent engineering advances have demonstrated synthetic apomixis in rice and Arabidopsis, but broader application in other crops has remained limited. In this study, researchers from Corteva Agriscience and The University of Queensland report the first successful induction of synthetic apomixis was successfully induced for the first time in hybrid sorghum, a predominantly self-pollinating crop in which access to hybrid seed is constrained by production costs and resource limitations. By combining CRISPR/Cas9-generated MiMe mutations with egg cell–specific expression of the ASGR-BBML2 gene, the authors generated self-reproducing (SR) hybrids capable of transmitting hybrid genotypes clonally through seed. Both one-step and two-step induction strategies proved effective across two hybrid backgrounds, with the two-step approach yielding more uniform parthenogenesis and the one-step method reducing transformation steps. Parthenogenesis induction reached ~40% in multiple lines, and 96.2% of SR progeny retained full heterozygosity, confirming robust clonal reproduction.
Despite this major advance, several biological constraints influencing apomictic efficiency were identified. Variation in parthenogenesis penetrance was associated with promoter choice, transgene insertion effects, and somaclonal variation, with the maize DD45 promoter driving stronger ASGR-BBML2 activity. Ploidy variation among progeny, including diploid, tetraploid, and rare aneuploid progeny, indicated incomplete meiotic non-reduction, likely reflecting functional complexity in the duplicated sorghum OsdL1 and OsdL3 genes, which are essential for both meiotic and mitotic cell cycle regulation. Seed set in synthetic apomicts remained lower than in sexual controls, consistent with observations in rice and highlighting the need to optimize embryo and endosperm development in engineered systems. Nonetheless, this study demonstrates that clonal hybrid sorghum is feasible, providing a critical foundation for future improvements that could enable scalable production of saveable, high-yielding hybrid seed for both commercial agriculture and smallholder farming systems.
SorghumBase Examples:
(A)
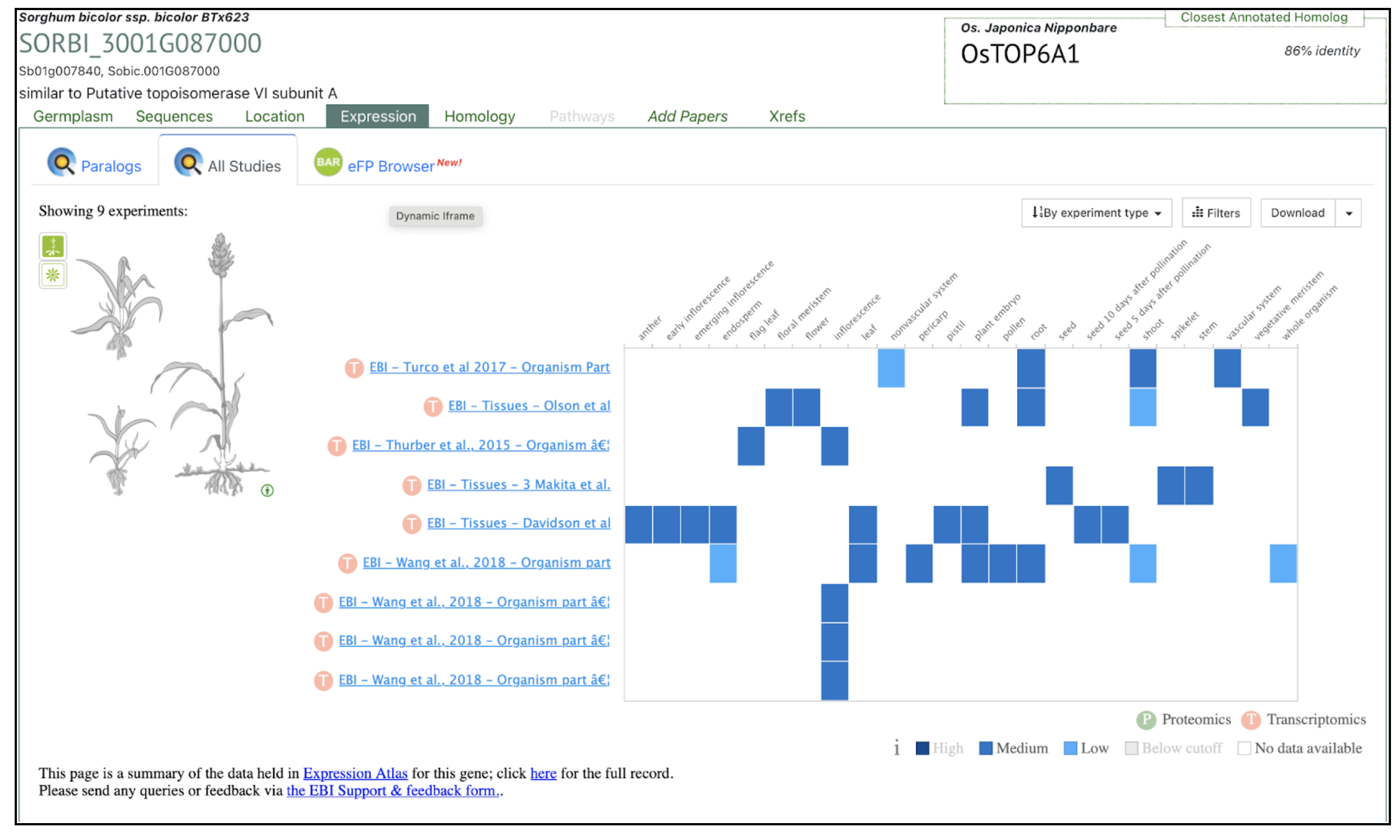
(B)
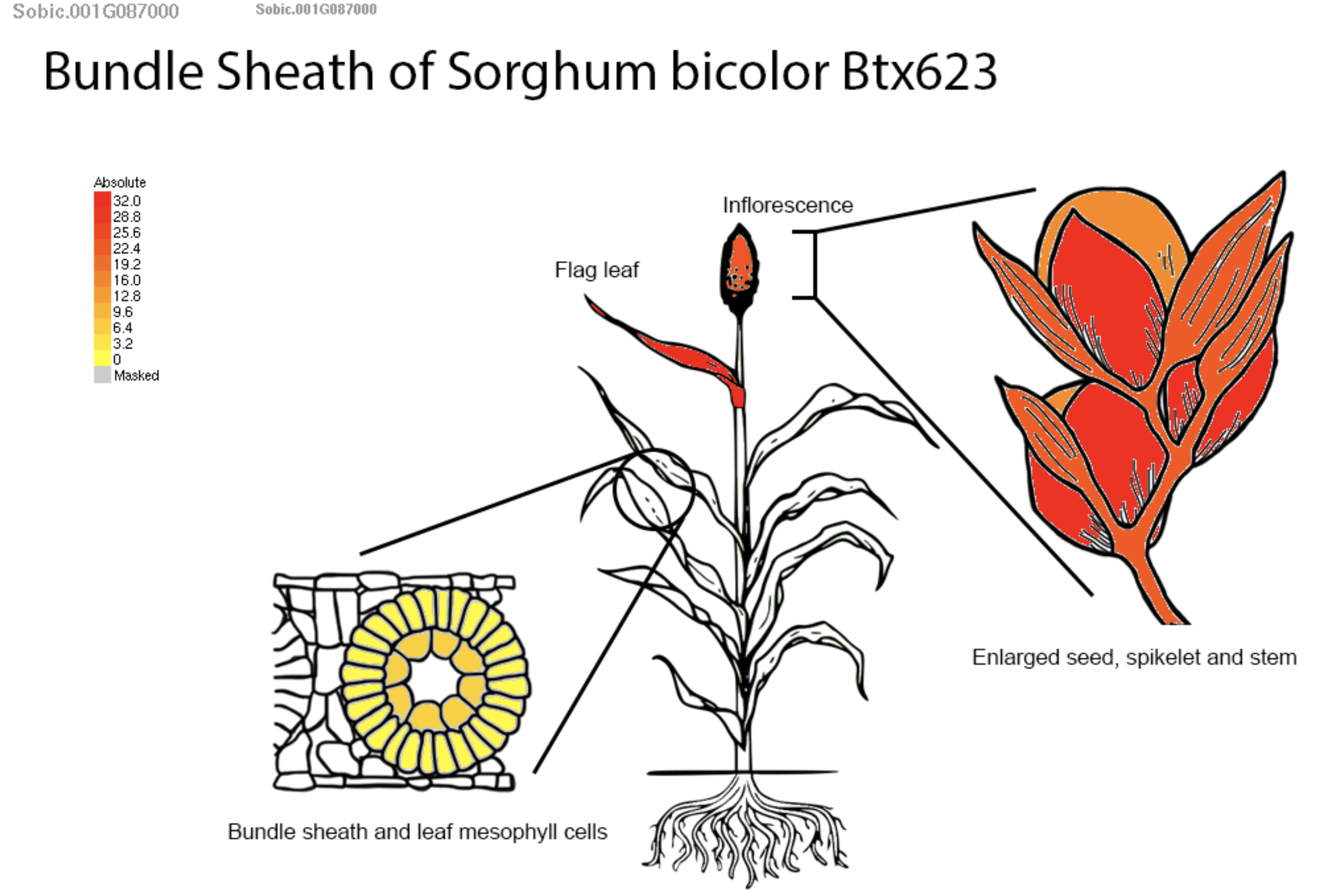
The SorghumBase Expression tab displays transcript abundance for SPO11-1 across nine datasets representing multiple tissues and developmental stages in BTx623. SPO11-1 shows distinctly elevated expression in anthers, floral tissues, and the floral meristem, whereas expression in vegetative tissues is low or declines over development. The accompanying eFP Browser visualization further localizes SPO11-1 expression to bundle sheath and mesophyll cell populations, providing fine-scale anatomical resolution. As a meiosis-specific endonuclease, SPO11-1 is known to initiate meiotic recombination by generating programmed DNA double-strand breaks, a critical step for homologous chromosome pairing and genetic diversity. Together, these expression profiles support findings from the study identifying SPO11-1 as enriched in reproductive tissues, consistent with its established meiotic function.
Reference:
Simon MK, Yuan L, Che P, Day K, Jones T, Godwin ID, Koltunow AMG, Albertsen MC. Induction of Synthetic Apomixis in Two Sorghum Hybrids Enables Seed Yield and Genotype Preservation Over Multiple Generations. Plant Biotechnol J. 2025 Nov 5.PMID: 41190715. doi: 10.1111/pbi.70441. Read more
Related Project Websites:
- Anna M G Koltunow’s page at The University of Queensland: https://qaafi.uq.edu.au/profile/6111/anna-koltunow
- Ian D Godwin’s page at The University of Queensland: https://about.uq.edu.au/experts/79
- Corteva Agriscience’s webpage: https://www.corteva.com/
- Hy-Gain Project Webpage: https://www.hy-gain.org

Photo Credit Megan Pope, UQ.