Anthracnose in sorghum (Sorghum bicolor (L.) Moench) is caused by the fungal pathogen Colletotrichum sublineola. Infection can cause reductions in yield of as much as 50%. The most economic and sustainable way to combat this pathogen is through the development of anthracnose-resistant cultivars. However, C. sublineola rapidly evolves and is genetically diverse, necessitating the integration of novel genetic sources of resistance into breeding programs. A genome-wide association study of the sorghum association panel previously found that Sobic.005G172300, which encodes an F-box protein, was a possible anthracnose resistance gene. In an effort to gain a better understanding of the way in which Sobic.005G172300 works to help plants defend against C. sublineola scientists from the University of Florida and the USDA-ARS monitored the gene expression post-infection through RNA-sequencing of both resistant (SC110) and susceptible sorghum lines. They initially determined that the gene expression increased in response to infection only in SC110. The researchers then used a transcriptomics approach to determine the molecular function of the gene, tracking the response over time and comparing the SC110 line with the three sorghum lines that are anthracnose susceptible, as well as mock-inoculated samples. Post-infection, SC110 sorghum showed a decrease in ascorbic acid, while levels of reactive oxygen species (ROS) increased. An increase in ROS has been associated with beginning defense signaling after pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) have been detected. Ascorbic acid functions as an antioxidant that detoxifies ROS and can modulate ROS levels through controlling its concentration. The researchers report that Sobic.005G172300 provides resistance to anthracnose through the regulation of ascorbic acid which, in turn, modulates the cellular concentration of ROS. Lower ascorbic acid amounts are associated with increased levels of ROS which help with cell signaling, and ultimately to the activation of pathogen response genes and localized programmed cell death. This suppresses early pathogen growth. Additionally, the researchers found that in SC110, the post-infection concentration of ROS was significantly higher than the concentration in mock-inoculated samples until 24 hours post infection, indicating an oxidative burst occurs during the early stages of infection for the SC110 sorghum plants. Taken together, these findings may contribute new genetic resources for breeding programs.
The majority of anthracnose resistance loci that have been characterized in sorghum so far are R-genes, encoding receptors that recognize the presence of C. sublineola, directly or indirectly. In the presence of the pathogen, these receptors will activate a defense response aimed at preventing fungal invasion or fungal death. The individual elements that make up this cascade are often unknown. The identification of a resistance gene encoding an F-box protein is surprising, because the F-box protein represents a part of the defense mechanism that is down-stream of the receptors. It reveals that having a functional receptor alone is not sufficient in mounting an effective defense response. This knowledge will help in the design of robust and durable resistance against a major pathogen of sorghum. – Wilfred Vermerris
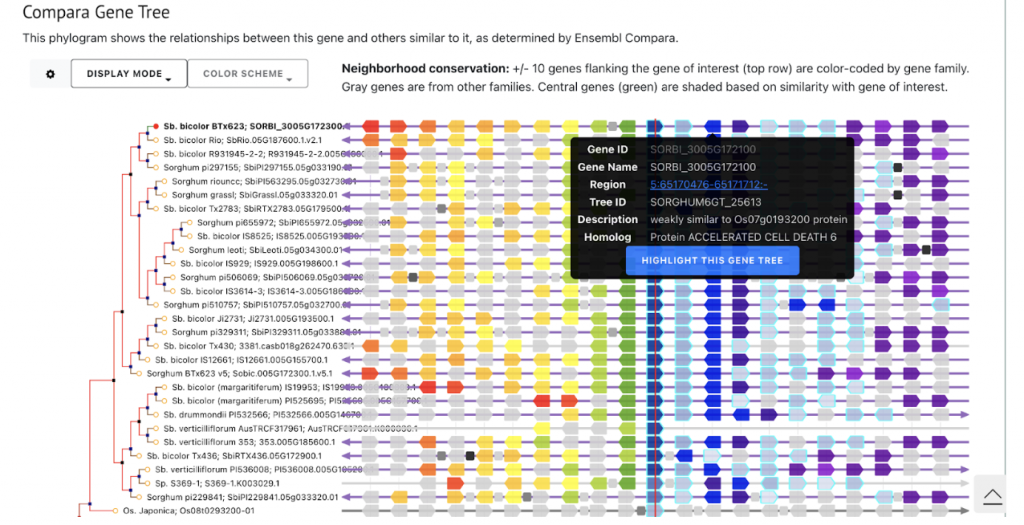
SorghumBase example:
Reference:
Wolf ESA, Vela S, Cuevas HE, Vermerris W. A sorghum F-box protein induces an oxidative burst in the defense against Colletotrichum sublineola. Phytopathology. 2023 Sep 17. PMID: 37717251. doi: 10.1094/PHYTO-06-23-0184-R. Read more
Related Project Websites:
Wilfred Vermerris page at the University of Florida: https://microcell.ufl.edu/people/wilfred-vermerris/
Other relevant publications on anthracnose resistance in sorghum can be accessed via the Google Scholar profiles of Dr. Vermerris and Dr. Cuevas:
- https://scholar.google.com/citations?hl=en&user=NEtVu0sAAAAJ
- https://scholar.google.com/citations?hl=en&user=0q2WeegAAAAJ&view_op=list_works&sortby=pubdate

